5 5 Dibromo 2 Methyloctane
1. Introduction
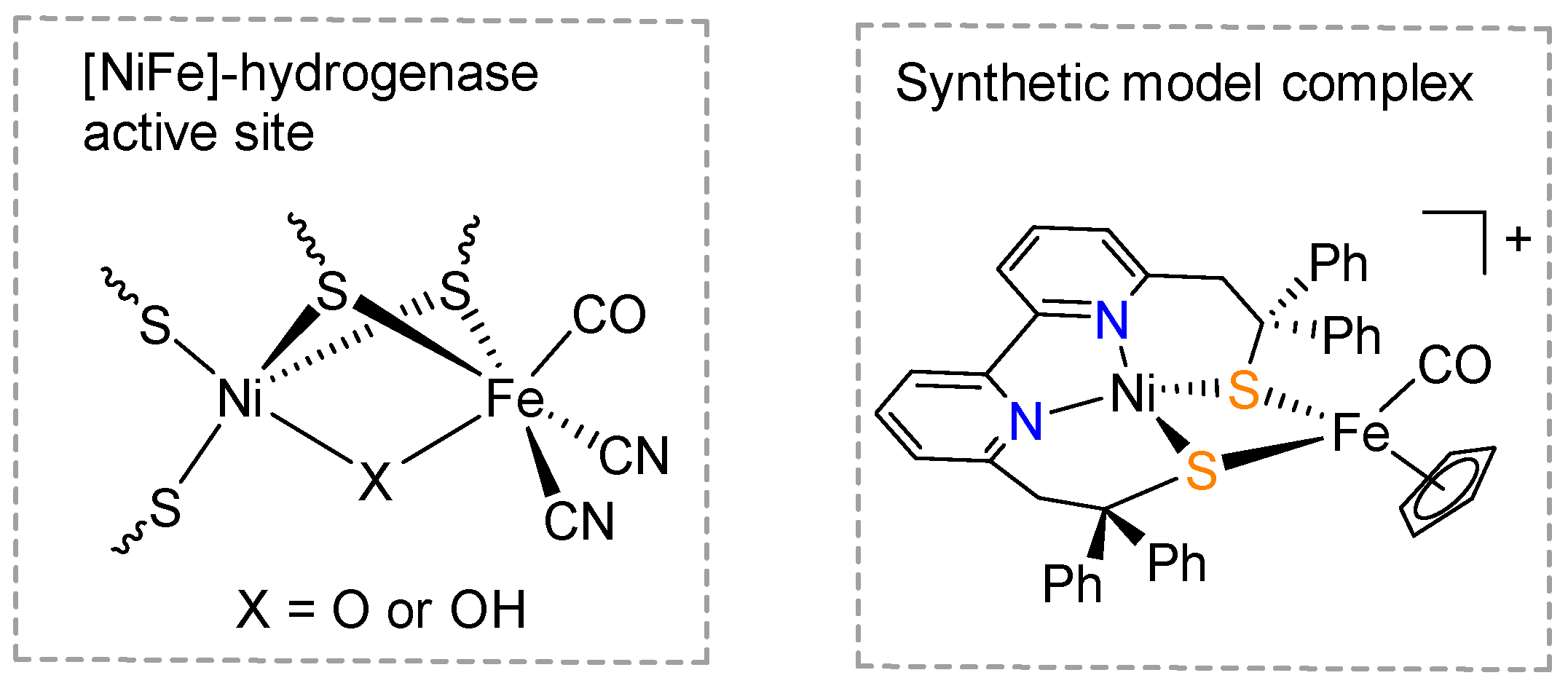
[NiFe]-hydrogenases in nature have the power of catalyzing the protons to hydrogen (Hii) reduction reaction at high rates with a small barrier of activation energy [1,ii]. The agile site of [NiFe]-hydrogenases is a bimetallic Ni-Atomic number 26 cluster, of which the Ni and Fe metallic centers are bridged past two cysteine rest thiolates (Scheme 1) [3,4,v]. Syntheses of metallic complexes that mimic the structure and function of the agile site of [NiFe]-hydrogenases have long been an important field of bioinorganic chemical science [6,7], and draw even more attending these days in the context of the evolution of a hydrogen economy [viii]. The biomimetic Ni and Iron model complexes can help us to understand the catalytic mechanism of hydrogenases, whilst also inspiring the pattern of transition element-based heterogeneous hydrogen development catalysts.
Artero et al. recently reported a heterodinuclear Ni-Fe circuitous (Scheme ane), namely LN2S2NiIIIronTwo, that models the active site of [NiFe]-hydrogenases and catalyzes electrochemical H2 evolution [nine,10,11]. This heterodinuclear complex was adult from the mononuclear nickel complex with the bipyridine-bisthiolate ligand, ii,2′-(2,two′-bipyridine-6,6′-diyl)bis(1,1-diphenylethanethiolate) [12,13]. Despite the successful preparation of LN2S2NiIiFeTwo as a unique and valuable model complex, artificial mimics for the active site of [NiFe] hydrogenase, with diverse structural features, are withal very rare. The central challenge for reproducing the [NiFe]-hydrogenases agile site in a synthetic system lies on the associates of multiple thiolate binding sites inside one organic ligand and, at the same time, in a pre-organized fashion. Here, nosotros report the synthesis of a novel organic ligand platform with bisthiophenol chelating donors, which has potential as a chelator of Ni cations in the awarding of syntheses of model complexes for [NiFe]-hydrogenases' active site.
ii. Results and Discussion
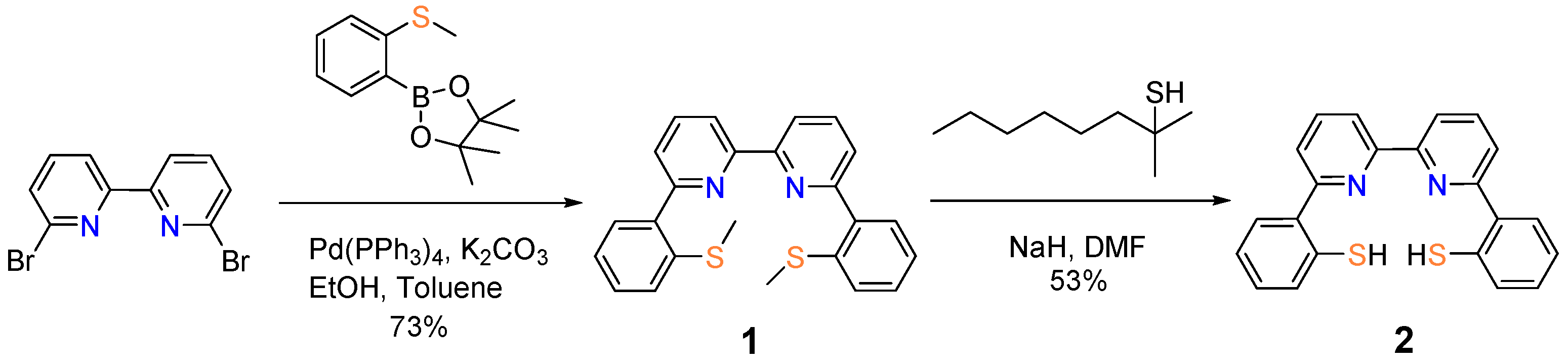
We designed the compound half-dozen,half dozen′-di-(2″-thiophenol)-ii,2′-bipyridine (2 in Scheme 2) past integrating the following 2 design features: (i) a rigid courage that provides analogous sites and regulates the coordination configuration at certain extent; (ii) the availability of multiple S− donors that mimic the coordination environment around the active site of [NiFe]-hydrogenase (Scheme 1). To the best of our knowledge, half dozen,6′-di-(2″-thiophenol)-2,2′-bipyridine (2) is the first case of tetradentate ligands that contain both bispyridine and bisthiophenol chelating moieties. A literature survey returned one striking of compound ii in a patent without synthetic details [14] An analogue of 1 with phenanthroline backbone has been reported earlier [xv].
The championship compound (two) was synthesized in a two-step procedure (Scheme two) from the commercially available starting material, 6,6′-dibromo-2,2′-bipyridine. Compound half dozen,half-dozen′-di-(2″-methylthiophenyl)-2,2′-bipyridine (1) was prepared under typical Suzuki–Miyaura coupling conditions using Pd(PPh3)4 as the catalyst and potassium carbonate as a base. The reaction went well in anaerobic toluene and afforded chemical compound 1 in a yield of 73%. Deprotection of the methyl groups was commencement performed with NaH and tert-nonyl mercaptan in DMF at 160 °C [16]. The conventional heating condition, however, did non effectively remove the thioether substituent. Given a relatively long reaction menses, TLC analysis of the reaction production revealed a collection of compounds without distinctive indication for the formation of ii. The awarding of a microwave reactor, which allows elevation of the reaction temperature to 200 °C, accomplished the target compound two in a reasonable yield (53%).
Compounds 1 and 2 were both characterized past 1H- and thirteenC-NMR spectroscopy. The proton NMR spectrum of 1 in CDCl3 shows the signal of methyl groups every bit a singlet at 2.44 ppm with the integration of 6H (Figure S1). This characteristic methyl proton peak disappears in the 1H-NMR spectrum of 2. Instead, a singlet with the integration of 2H emerges at 4.57 ppm (Effigy S3) and is assigned as the thiophenol protons. Elemental analysis was conducted to verify the purity of compounds ane and 2. A high-resolution mass spectrometer was also employed to confirm the molecular formula of ane (Figure S5). Comparing the FT-IR spectra of 1 and 2 (Figure S6) reveals the South−H stretching bands at 2506 and 2530 cm−i, which are close to the Due south−H stretching band of thiophenol (2545 cm−1) [17].
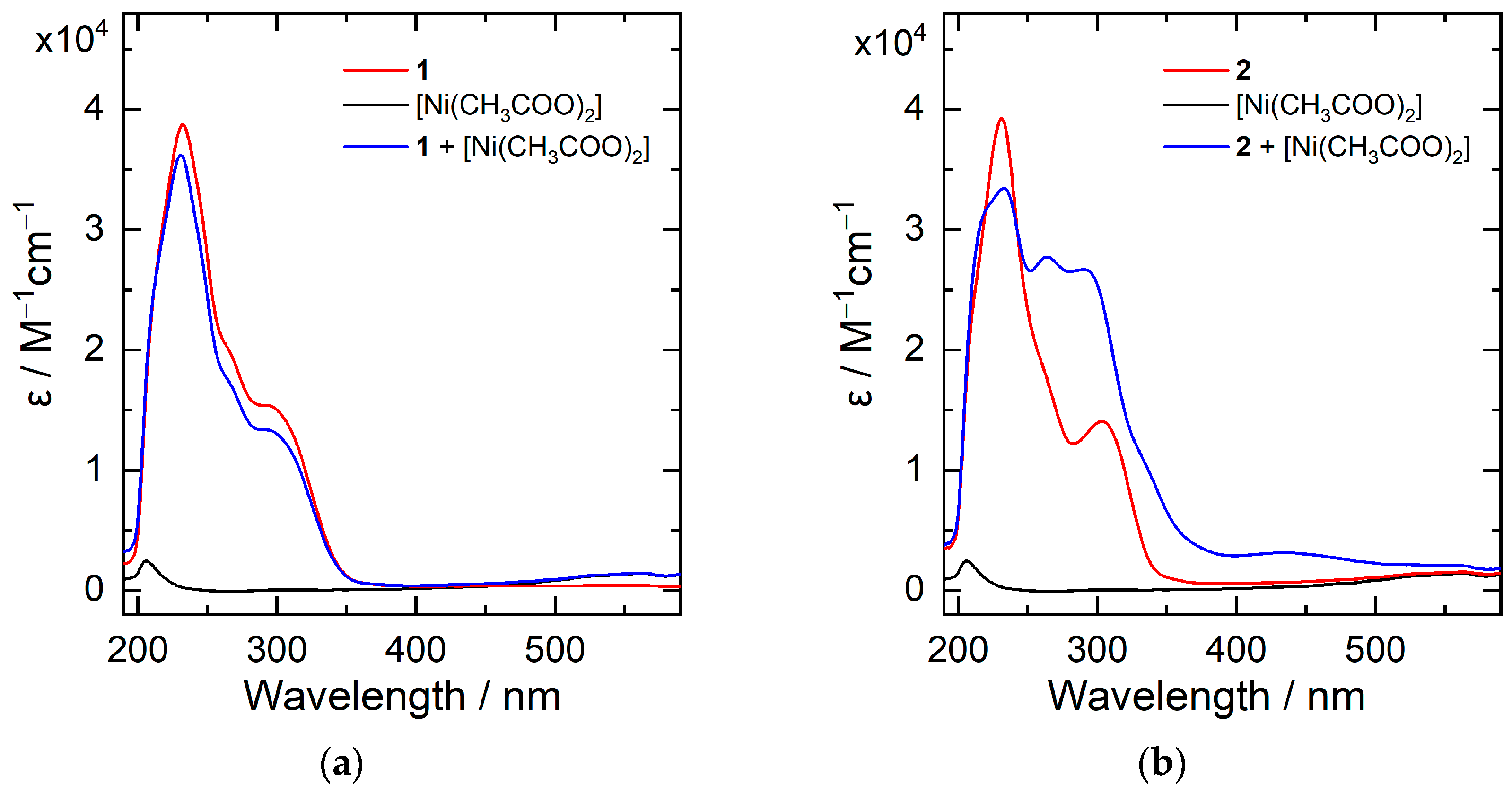
The UV-Vis spectra of one and 2 were recorded in methanol, every bit displayed in Figure 1. Both compounds bear witness strong absorbance bands at λ max = 231 and 303 nm, which derive from the π → π* electron excitation at the pyridyl and phenyl moieties. The addition of one equivalent nickel acetate in the methanol solution of ii results in significant modify of the UV-Vis absorption contour: the emergence of absorbance bands at λ max = 263 and 291 nm. The phenomena suggest coordination of Ni(II) ion by the tetradentate compound ii. In contrast, the UV-Vis spectrum of one is not affected by the presence of nickel ion, indicating weak or no interaction betwixt the chemical compound Ni(II) in solution. Synthesis and isolation of 3d-transition element complexes, peculiarly Ni and Iron complexes, with compound 2 every bit a ligand are being carried out.
three. Materials and Methods
All air- and moisture-sensitive experiments were performed under a dry argon temper using standard Schlenk techniques. Dry solvents for moisture-sensitive experiments were purchased from commercial sources (water content ≤ ten ppm) and used as received without farther purification. six,half dozen′-dibromo-two,2′-bipyridine, 4,4,five,five-tetramethyl-2-(two-(methylthio)phenyl)-1,three,2-dioxaborolane, two-methyloctane-2-thiol, and other chemicals for syntheses were commercially available and used as received. Microwave syntheses were carried out using an Anton-Parr Monowave 200 microwave reactor (Anton-Parr, Graz, Austria). Water for syntheses and assay was purified by Milli-Q technique (18.2 MΩ, Merck, Darmstadt, Deutschland). Thin Layer Chromatography analyses were performed on silica gel coated glass plates with fluorescence indicator UV254. Flash column chromatography was conducted with silica gel at atmospheric pressure.
aneH- and 13C-NMR spectra were recorded on a Bruker (Fällanden, Switzerland) Avance NEO (600 MHz) spectrometer, operating at a probe temperature of room temperature. Chemical shifts, δ, are reported in ppm relative to the summit of SiMe4, using 1H chemical shifts of the residual solvents as references [eighteen]. Electronic absorption spectra were recorded with a meaty OTO Photonics (Hsinchu, Taiwan) UV-Vis spectrometer (SE2030-050-FUV). Loftier-resolution MS information were obtained using an Agilent (Santa Clara, CA, U.s.a.) 1260-6460 Q-TOF mass spectrometer. FT-IR spectra were caused using the TENSOR Two + Hyperion 2000 spectroscopy (Bruker, Ettlingen, Germany). Elemental analysis (C North H S) was performed on Vario EL Cube (Elementar, Langenselbold, Germany).
-
Synthesis of half dozen,6′-di-(ii″-methylthiophenyl)-2,two′-bipyridine (1).
4,4,five,five-Tetramethyl-2-(2-(methylthio)phenyl)-1,3,two-dioxaborolane (1.0 g, 4.0 mmol) was added to a solution of 6,half dozen′-dibromo-2,ii′-bipyridine (313 mg, 1.0 mmol) in a mixture of toluene (seven mL) and EtOH (7 mL). After degassing by Ar, K2COthree (four.xiv g, 30 mmol) and Pd(PPh3)iv (58 mg, 0.05 mmol) were added to this solution and the mixture was heated by a microwave reactor to 170 °C for 65 min under stirring. The solution was allowed to cool to room temperature and the volatile components were removed nether vacuum. The residue was extracted with methylene chloride (fifty mL × 3) iii times. The combined organic layers were washed by saturated sodium chloride solution and then dried by anhydrous sodium sulfate. The solid salt was removed past filtration. Removal of the solvent under vacuum afforded chemical compound 1 as an orange powder (292 mg. 73%). 1H-NMR (600 MHz, Chloroform-d) δ 8.61 (dd, J = 7.9, 1.0 Hz, 2H), 7.ninety (t, J = 7.eight Hz, 2H), 7.58 (ddd, J = 10.six, 7.5, one.two Hz, 4H), vii.43–seven.38 (k, 4H), vii.29–7.26 (m, 2H), ii.44 (due south, 6H). 13C-NMR (151 MHz, Chloroform-d) δ 157.59, 155.43, 138.23, 137.45, 130.ten, 129.02, 126.29, 124.88, 123.77, 120.11, 77.16, 16.92. ESI-HRMS: m/eastward calcd for C24H21North2Due south2 (1000 + H)+ 401.1146, found 401.1146. Mp: 215–218 °C. Anal. Calcd. for ane (C24H20N2S2): C, 71.97; H, 5.03; Northward, vi.99; Southward, xvi.01. Found: C, 71.65; H, five.05; North, 6.73; S, 15.77.
-
Synthesis of 6,6′-di-(two″-thiophenol)-2,ii′-bipyridine (2).
2-Methyloctane-2-thiol (640 mg, 4.0 mmol) was added to a solution of NaH (96 mg, 4.0 mmol) in anhydrous DMF (xiii mL). Compound 1 (200 mg, 0.5 mmol) was added to this solution and the mixture was stirred under an Ar atmosphere for about 10 min, until the gas bubbles ceased. The mixture was then transferred to a drinking glass tube (designed for microwave reaction) and heated by a microwave reactor to 200 °C for 75 min under stirring. The solution was allowed to cool to room temperature, and so diluted hydrochloric acid (25 mL) was slowly dropped into it. The orangish precipitate was collected by filtration and purified by column chromatography over silica using CH2Cl2 as an eluent. The pure product was obtained as an orange powder (98 mg, 53%). aneH-NMR (600 MHz, Chloroform-d) δ 8.64 (dd, J = seven.8, 1.0 Hz, 2H), 7.95 (t, J = 7.8 Hz, 2H), seven.64–7.58 (yard, 4H), 7.48–7.43 (g, 2H), 7.29–vii.26 (chiliad, 4H), four.57 (s, 2H). 13C-NMR (151 MHz, Chloroform-d) δ 138.18, 137.92, 132.21, 131.37, 130.41, 129.01, 125.73, 123.55, 120.03, 77.16. Mp: 179–182 °C. Anal. Calcd. for 2· (C22H16North2Stwo): C, 70.94; H, iv.33; Northward, 7.52; S, 17.21. Anal. Calcd. for 2·0.6H2O (C22H17.2NorthwardiiO0.6South2): C, 68.94; H, 4.52; N, 7.31; South, sixteen.73. Institute: C, 68.64; H, 4.35; Due north, half dozen.87; S, 16.87.
four. Conclusions
Compounds di-(2″-methylthiophenyl)-2,ii′-bipyridine (1) and half dozen,half-dozen′-di-(ii″-thiophenol)-2,ii′-bipyridine (2) have been prepared and characterized. The deprotection of methyl groups of one with tert-nonyl mercaptan was achieved in DMF using a microwave reactor at 200 °C. The thiophenol bipyridine compound 2 might be used every bit a chelator for the Ni cation.
Supplementary Materials
The following supporting information can exist downloaded: NMR spectra and HRMS analysis. Effigy S1. 1H-NMR spectrum of compound 1 in CDCl3. Figure S2. 13C{1H} NMR spectrum of compound ane in CDCliii. Figure S3. aneH-NMR spectrum of chemical compound 2 in CDClthree. Figure S4. 13C{1H} NMR spectrum of compound two in CDCl3. Figure S5. HRMS spectrum of chemical compound 1. Figure S6. FT-IR spectra of compounds 1 (blue) and two (red) every bit KBr pellets.
Author Contributions
Y.H. and 50.T. conceived and designed the synthetic route, analyzed the information, and drafted the manuscript. Y.H. conducted the experiments of syntheses and characterization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Guangzhou University nether the funding number RQ2020042.
Information Availability Statement
The information are reported in the manuscript and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jones, A.Thou.; Sillery, Due east.; Albracht, South.P.; Armstrong, F.A. Direct comparison of the electrocatalytic oxidation of hydrogen past an enzyme and a platinum catalyst. Chem. Commun. 2002, 866–867. [Google Scholar] [CrossRef] [PubMed]
- Frey, M. Hydrogenases: Hydrogen-Activating Enzymes. ChemBioChem 2002, iii, 153–160. [Google Scholar] [CrossRef]
- Volbeda, A.; Charon, M.-H.; Piras, C.; Hatchikian, E.C.; Frey, M.; Fontecilla-Camps, J.C. Crystal structure of the nickel-iron hydrogenase from Desulfovibrio gigas. Nature 1995, 373, 580. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, J.; Scheerer, P.; Frielingsdorf, S.; Kroschinsky, Due south.; Friedrich, B.; Lenz, O.; Spahn, C.K.T. The crystal structure of an oxygen-tolerant hydrogenase uncovers a novel fe-sulphur middle. Nature 2011, 479, 249. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Nishikawa, K.; Lubitz, W. Hydrogens detected by subatomic resolution protein crystallography in a [NiFe] hydrogenase. Nature 2015, 520, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.R.; Berggren, 1000.; Bacchi, G.; Fontecave, M.; Artero, 5. Mimicking hydrogenases: From biomimetics to bogus enzymes. Coord. Chem. Rev. 2014, 270–271, 127–150. [Google Scholar] [CrossRef]
- Ahmed, Thou.Eastward.; Dey, A. Contempo developments in bioinspired modelling of [NiFe]- and [FeFe]-hydrogenases. Curr. Opin. Electrochem. 2019, 15, 155–164. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, Northward.; Ward, 1000.R. The part of hydrogen and fuel cells in the global free energy organization. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef][Dark-green Version]
- Brazzolotto, D.; Gennari, M.; Queyriaux, North.; Simmons, T.R.; Pecaut, J.; Demeshko, S.; Meyer, F.; Orio, G.; Artero, Five.; Duboc, C. Nickel-centred proton reduction catalysis in a model of [NiFe] hydrogenase. Nat. Chem. 2016, eight, 1054–1060. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahmed, M.Due east.; Chattopadhyay, S.; Wang, L.; Brazzolotto, D.; Pramanik, D.; Aldakov, D.; Fize, J.; Morozan, A.; Gennari, One thousand.; Duboc, C.; et al. Hydrogen Evolution from Aqueous Solutions Mediated past a Heterogenized [NiFe]-Hydrogenase Model: Low pH Enables Catalysis through an Enzyme-Relevant Machinery. Angew. Chem. Int. Ed. 2018, 57, 16001–16004. [Google Scholar] [CrossRef] [PubMed]
- Brazzolotto, D.; Wang, L.; Tang, H.; Gennari, M.; Queyriaux, Northward.; Philouze, C.; Demeshko, Due south.; Meyer, F.; Orio, M.; Artero, V.; et al. Tuning Reactivity of Bioinspired [NiFe]-Hydrogenase Models by Ligand Blueprint and Modeling the CO Inhibition Process. ACS Catal. 2018, viii, 10658–10667. [Google Scholar] [CrossRef]
- Gennari, G.; Orio, M.; Pecaut, J.; Neese, F.; Collomb, 1000.N.; Duboc, C. Reversible upmost coordination of imidazole betwixt the Ni(Three) and Ni(II) oxidation states of a dithiolate complex: A procedure related to the Ni superoxide dismutase. Inorg. Chem. 2010, 49, 6399–6401. [Google Scholar] [CrossRef] [PubMed]
- Hamacher, C.; Hurkes, Northward.; Kaiser, A.; Klein, A.; Schuren, A. Electrochemistry and spectroscopy of organometallic terpyridine nickel complexes. Inorg. Chem. 2009, 48, 9947–9951. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K. White Organic Electroluminescence Device. JP Patent JP2010135689A, 17 June 2010. [Google Scholar]
- Neuhaus, J.D.; Morrow, Southward.M.; Brunavs, M.; Willis, One thousand.C. Diversely Substituted Quinolines via Rhodium-Catalyzed Alkyne Hydroacylation. Org. Lett. 2016, eighteen, 1562–1565. [Google Scholar] [CrossRef][Greenish Version]
- Manes, T.A.; Rose, Thou.J. Mono- and Dinuclear Manganese Carbonyls Supported by ane,viii-Disubstituted (L = Py, SMe, SH) Anthracene Ligand Scaffolds. Inorg. Chem. 2016, 55, 5127–5138. [Google Scholar] [CrossRef]
- Kim, H.J.; Yoon, J.H.; Yoon, S. Photooxidative coupling of thiophenol derivatives to disulfides. J. Phys. Chem. A 2010, 114, 12010–12015. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.G.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef][Green Version]
Scheme i. A schematic representation of the active site of [NiFe]-hydrogenase (left) and the molecular structure of a synthetic model (LN2S2NiIIIronII, correct) of [NiFe]-hydrogenase [9].
Scheme i. A schematic representation of the active site of [NiFe]-hydrogenase (left) and the molecular structure of a constructed model (50N2S2NiIIAtomic number 26II, right) of [NiFe]-hydrogenase [9].
Scheme 2. Synthesis of half-dozen,6′-di-(2″-thiophenol)-two,2′-bipyridine.
Scheme 2. Synthesis of vi,six′-di-(2″-thiophenol)-two,2′-bipyridine.
Effigy 1. UV-Vis spectra of 1 (a) and 2 (b) measured in methanol in the absenteeism and presence of 1 equivalent of Ni(II) acetate.
Figure one. UV-Vis spectra of 1 (a) and two (b) measured in methanol in the absenteeism and presence of one equivalent of Ni(II) acetate.
| Publisher'southward Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed nether the terms and atmospheric condition of the Creative Eatables Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
5 5 Dibromo 2 Methyloctane,
Source: https://www.mdpi.com/1422-8599/2022/2/M1355/htm
Posted by: johansenunly1998.blogspot.com


0 Response to "5 5 Dibromo 2 Methyloctane"
Post a Comment